Summary
- Description
- Getting ready
- Prepare data
- Prepare user pre-processed data
- Using Custom Data Partitions
- Analysis of extrapolation risks in partitions
- Saving a prepared_data object
Description
Before starting the ENM process, data must be formatted in a specific
structure required by functions in kuenm2. This
vignette guides users through the steps necessary to prepare occurrence
data and environmental predictors using built-in tools. It covers the
use of prepare_data() and prepare_user_data()
to generate standardized objects, which are essential for model
calibration. The vignette also demonstrates options for applying PCA,
incorporating sampling bias, and saving prepared data for later use.
Getting ready
If kuenm2 has not been installed yet, please do so. See the Main guide for installation instructions. See the basic data cleaning guide for some steps on cleaning data.
Use the following lines of code to load kuenm2 and any other required packages, and define a working directory (if needed). In general, setting a working directory in R is considered good practice, as it provides better control over where files are read from or saved to. If users are not working within an R project, we recommend setting a working directory, since at least one file will be saved at later stages of this guide.
# Load packages
library(kuenm2)
library(terra)
# Current directory
getwd()
# Define new directory
#setwd("YOUR/DIRECTORY") # uncomment this line if setting a new directoryPrepare data
Import data
We will use occurrence records provided within the
kuenm2 package. Most example data in the package is
derived from Trindade &
Marques (2024). The occ_data object contains 51
occurrences of Myrcia hatschbachii, a tree endemic to Southern
Brazil. Although this example data set has three columns (species, x,
and y), users’ input data only requires two numeric columns with
longitude and latitude coordinates.
# Import occurrences
data(occ_data, package = "kuenm2")
# Check data structure
str(occ_data)
#> 'data.frame': 51 obs. of 3 variables:
#> $ species: chr "Myrcia hatschbachii" "Myrcia hatschbachii" "Myrcia hatschbachii" "Myrcia hatschbachii" ...
#> $ x : num -51.3 -50.6 -49.3 -49.8 -50.2 ...
#> $ y : num -29 -27.6 -27.8 -26.9 -28.2 ...As predictor variables, we will use other data included in the package. This data set comprises four bioclimatic variables from WorldClim 2.1 at 10 arc-minute resolution, and a categorical variable (SoilType) from SoilGrids resampled to 10 arc-minutes. All variables have been masked using a polygon that delimits the area for model calibration, which was generated by drawing a minimum convex polygon around the records with a 300 km buffer.
# Import raster layers
var <- rast(system.file("extdata", "Current_variables.tif", package = "kuenm2"))
# Check variables
plot(var)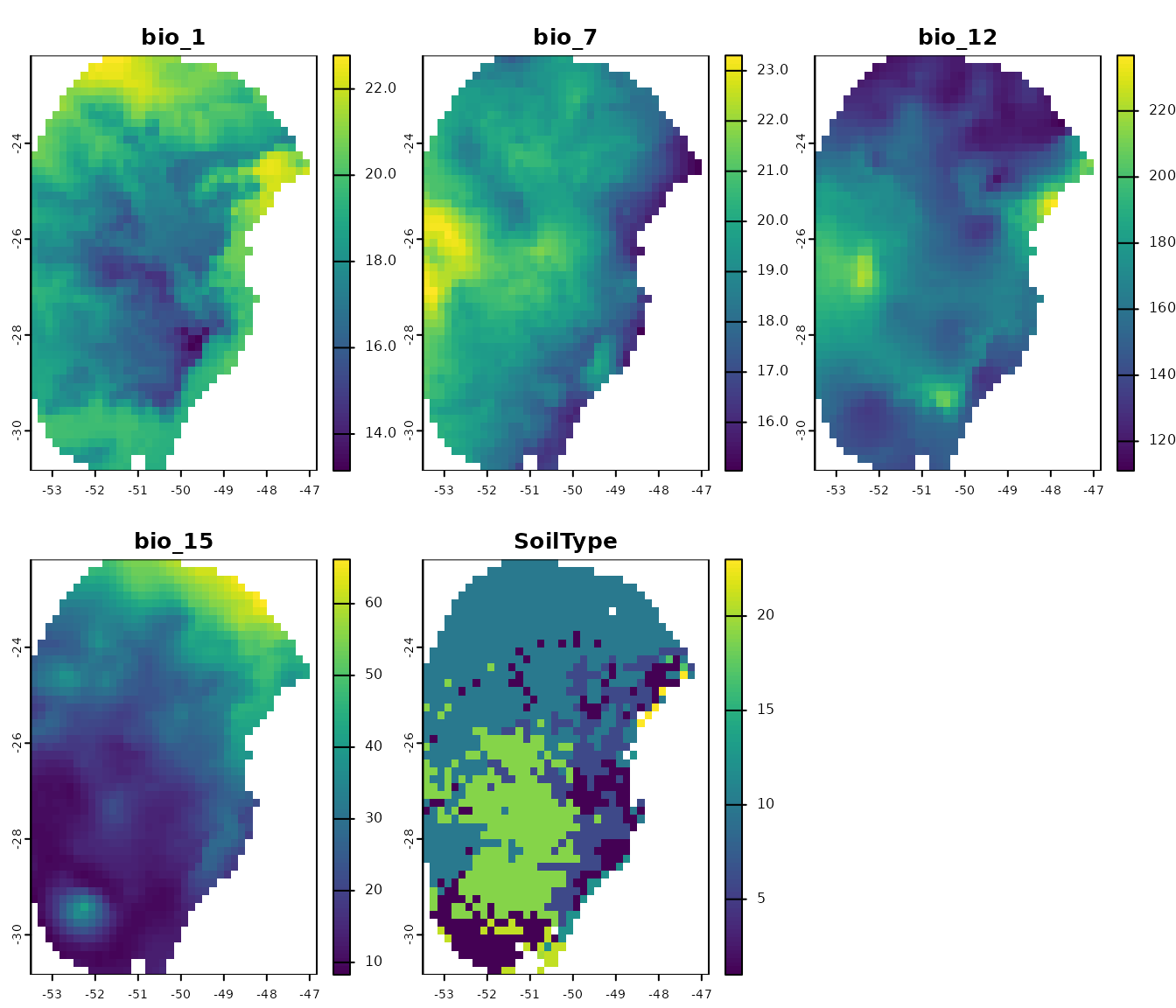
Visualize occurrences records in geography:
# Visualize occurrences on one variable
plot(var[["bio_1"]], main = "Bio 1")
points(occ_data[, c("x", "y")], col = "black")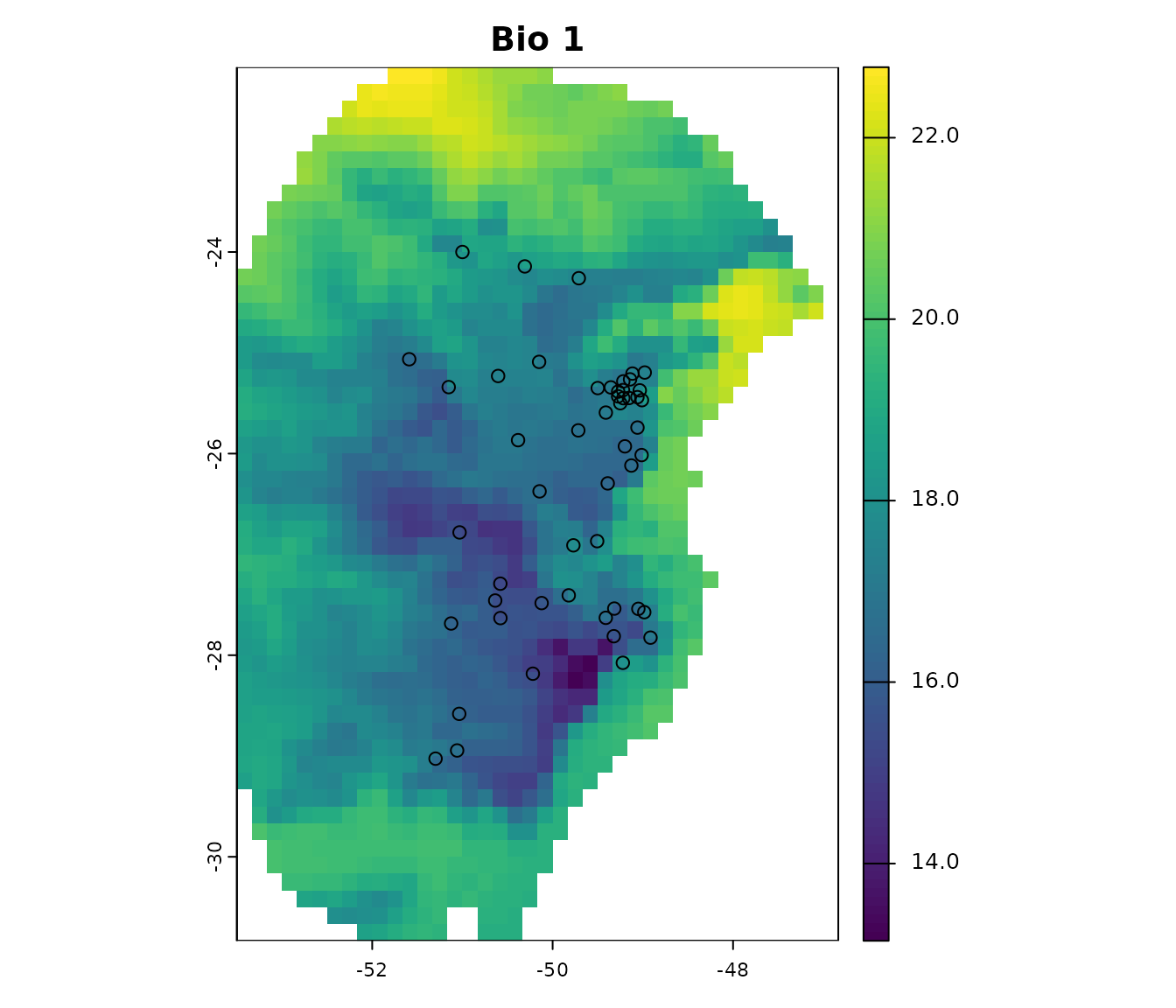
First steps in preparing data
The function prepare_data() is central to getting data
ready for model calibration. It handles several key steps:
-
Defining the algorithm: Users can choose between
maxnetorglm. - Generating background points: Background points area sampled from raster layers, unless provided by the user. These points serve as a reference to contrast presence records.
- Principal component analysis (PCA): An optional step that can be applied to the set of predictors in PCA.
-
Preparing calibration data: Presence records and
background points are associate with predictor values and put together
in a
data.frameto be used in the ENM. -
Data partitioning: The function divides your data
to prepare training and testing sets via a cross-validation process. The
partitioning methods available includes
kfolds,subsample, andbootstrap. - Defining grid of model parameters: This helps setting up combinations of feature classes (FCs), regularization multiplier (RM) values (for Maxnet), and sets of predictor variables. An explanation of the roles of RMs and FCs in Maxent models see Merow et al. 2013.
As with any function, we recommend consulting the documentation for
more detailed explanations (e.g., help(prepare_data)). Now,
let’s prepare our data for model calibration using
prepare_data(), using 4 k-folds as the partitioning
method:
# Prepare data for maxnet model
d <- prepare_data(algorithm = "maxnet",
occ = occ_data,
x = "x", y = "y",
raster_variables = var,
species = "Myrcia hatschbachii",
categorical_variables = "SoilType",
partition_method = "kfolds",
n_partitions = 4,
n_background = 1000,
features = c("l", "q", "lq", "lqp"),
r_multiplier = c(0.1, 1, 2))
#> Warning in handle_missing_data(occ_bg, weights): 43 rows were excluded from
#> database because NAs were found.The prepare_data() function returns a
prepared_data object, which is a list containing various
essential pieces of information fro model calibration. Below is an
example of how the object is printed to summarize its components.
print(d)
#> prepared_data object summary
#> ============================
#> Species: Myrcia hatschbachii
#> Number of Records: 1008
#> - Presence: 51
#> - Background: 957
#> Partition Method: kfolds
#> - Number of kfolds: 4
#> Continuous Variables:
#> - bio_1, bio_7, bio_12, bio_15
#> Categorical Variables:
#> - SoilType
#> PCA Information: PCA not performed
#> Weights: No weights provided
#> Calibration Parameters:
#> - Algorithm: maxnet
#> - Number of candidate models: 300
#> - Features classes (responses): l, q, lq, lqp
#> - Regularization multipliers: 0.1, 2, 1The parts of the prepared_data object can be explored in
further detail by indexing them as in the following example.
# See first rows of calibration data
head(d$calibration_data)
#> pr_bg bio_1 bio_7 bio_12 bio_15 SoilType
#> 1 1 16.49046 18.66075 1778 12.96107 19
#> 2 1 15.46644 19.65775 1560 14.14697 19
#> 3 1 15.70560 17.99450 1652 23.27548 6
#> 4 1 17.78899 19.55600 1597 18.91694 1
#> 5 1 15.50116 18.30750 1497 15.39440 19
#> 6 1 17.42421 17.25875 1760 34.17664 6
# See first rows of formula grid
head(d$formula_grid)
#> ID Formulas R_multiplier Features
#> 1 1 ~bio_1 + bio_7 -1 0.1 l
#> 2 2 ~bio_1 + bio_7 -1 2.0 l
#> 3 3 ~bio_1 + bio_7 -1 1.0 l
#> 4 4 ~bio_1 + bio_12 -1 2.0 l
#> 5 5 ~bio_1 + bio_12 -1 1.0 l
#> 6 6 ~bio_1 + bio_12 -1 0.1 lBy default, prepare_data() prepares an object for
fitting models using maxnet. However, this can be changed
to use GLM instead. When using GLM, it is not necessary to set
regularization multipliers, as this algorithm does not utilize them.
Let’s create an object for fitting models using glm,
this time using the subsample partitioning method, with 10
replicates and 70% of the dataset used for training.
d_glm <- prepare_data(algorithm = "glm",
occ = occ_data,
x = "x", y = "y",
raster_variables = var,
species = "Myrcia hatschbachii",
categorical_variables = "SoilType",
partition_method = "subsample",
n_partitions = 10,
train_proportion = 0.7,
n_background = 300,
features = c("l", "q", "p", "lq", "lqp"),
r_multiplier = NULL) #Not necessary with glms
#> Warning in handle_missing_data(occ_bg, weights): 8 rows were excluded from
#> database because NAs were found.
#Print object
d_glm
#> prepared_data object summary
#> ============================
#> Species: Myrcia hatschbachii
#> Number of Records: 343
#> - Presence: 51
#> - Background: 292
#> Partition Method: subsample
#> - Number of replicates:
#> - Train proportion: 0.7
#> Continuous Variables:
#> - bio_1, bio_7, bio_12, bio_15
#> Categorical Variables:
#> - SoilType
#> PCA Information: PCA not performed
#> Weights: No weights provided
#> Calibration Parameters:
#> - Algorithm: glm
#> - Number of candidate models: 122
#> - Features classes (responses): l, q, p, lq, lqpBy default, prepare_data() extracts background points
from the entire extent of the provided raster variables. If you have a
species-specific polygon defining the calibration area, you can use it
to mask the raster variables and delimit the calibration area. For
example, let’s create a 100km buffer around the occurrence records:
#Convert dataframe with occurrences to SpatVector
pts <- vect(occ_data, geom = c(x = "x", y = "y"), crs = "EPSG:4326")
#Create buffer
b <- buffer(x = pts, width = 100000) #Width in meters
#Aggregate buffers
b <- terra::aggregate(b)
#Plot buffer
plot(b)
points(occ_data[, c("x", "y")], col = "black")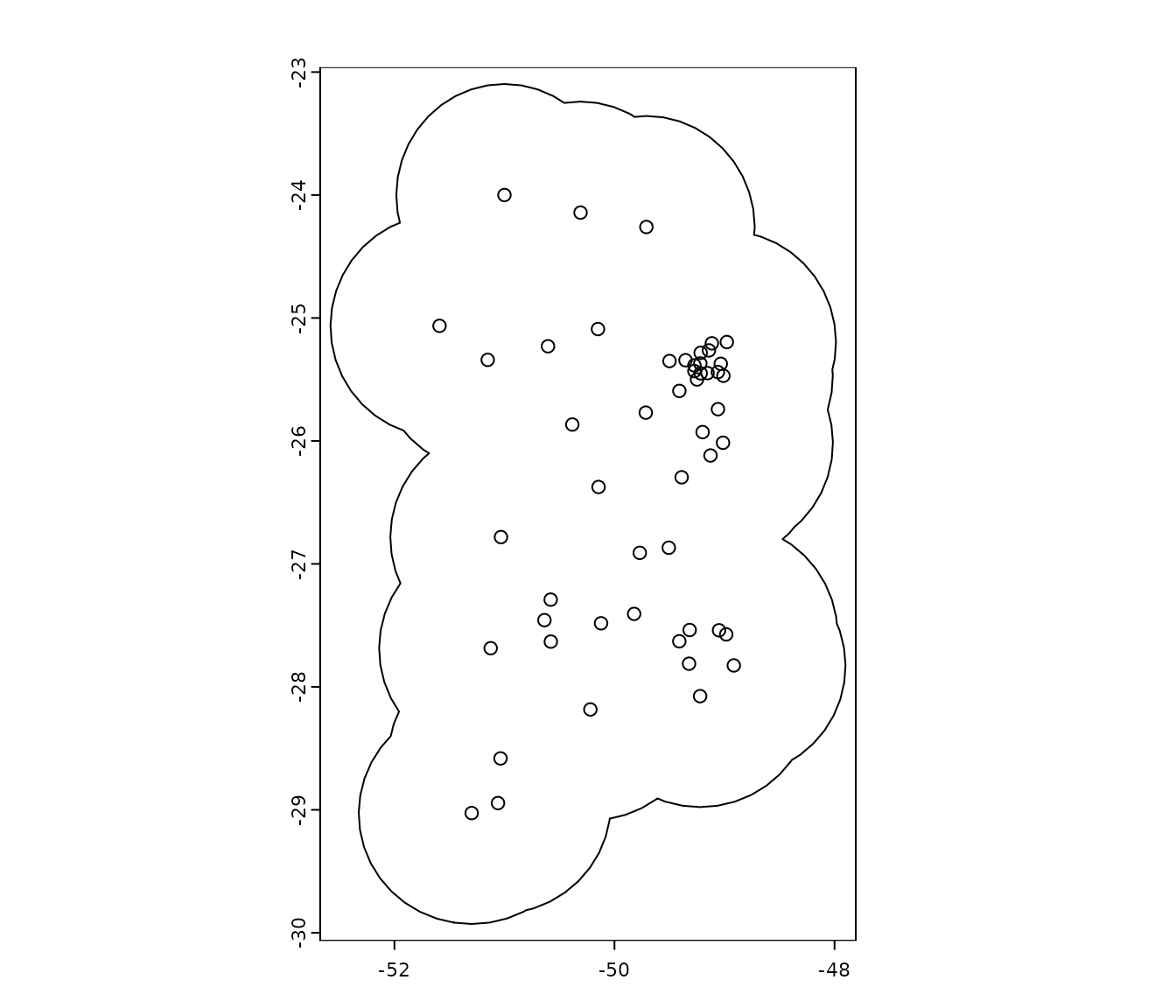
Now, let’s use this new polygon to mask the variables within
prepare_data(). Let’s also increase the number of
background points to 1,000:
d_buffer <- prepare_data(algorithm = "maxnet",
occ = occ_data,
x = "x", y = "y",
raster_variables = var,
mask = b, #Polygon to mask variables
species = "Myrcia hatschbachii",
categorical_variables = "SoilType",
n_background = 1000, partition_method = "kfolds",
features = c("l", "q", "p", "lq", "lqp"),
r_multiplier = c(0.1, 1, 2, 3, 5))
#> 'n_background' >= initial number of points, using all points.
#> Warning in handle_missing_data(occ_bg, weights): 25 rows were excluded from
#> database because NAs were found.Note the warning message: “n_background’ >= initial number of points, using all points”. This occurs because, after masking the variables, the total number of pixels within the buffer is less than the specified n_background (1,000). In such cases, all available points within the calibration area will be used as background points.
In the following examples, we’ll use the object
d_maxnet, which was prepared for the maxnet algorithm
without a mask. However, all functions are compatible with objects
prepared for GLM as well.
Exploring calibration data
Users can visualize the distribution of predictor values for
occurrence records, background points, and the entire calibration area
using histograms. An example is presented below. See full documentation
with help(explore_calibration_hist) and
help(plot_explore_calibration).
# Prepare histogram data
calib_hist <- explore_calibration_hist(data = d, raster_variables = var,
include_m = TRUE)
# Plot histograms
plot_explore_calibration(explore_calibration = calib_hist)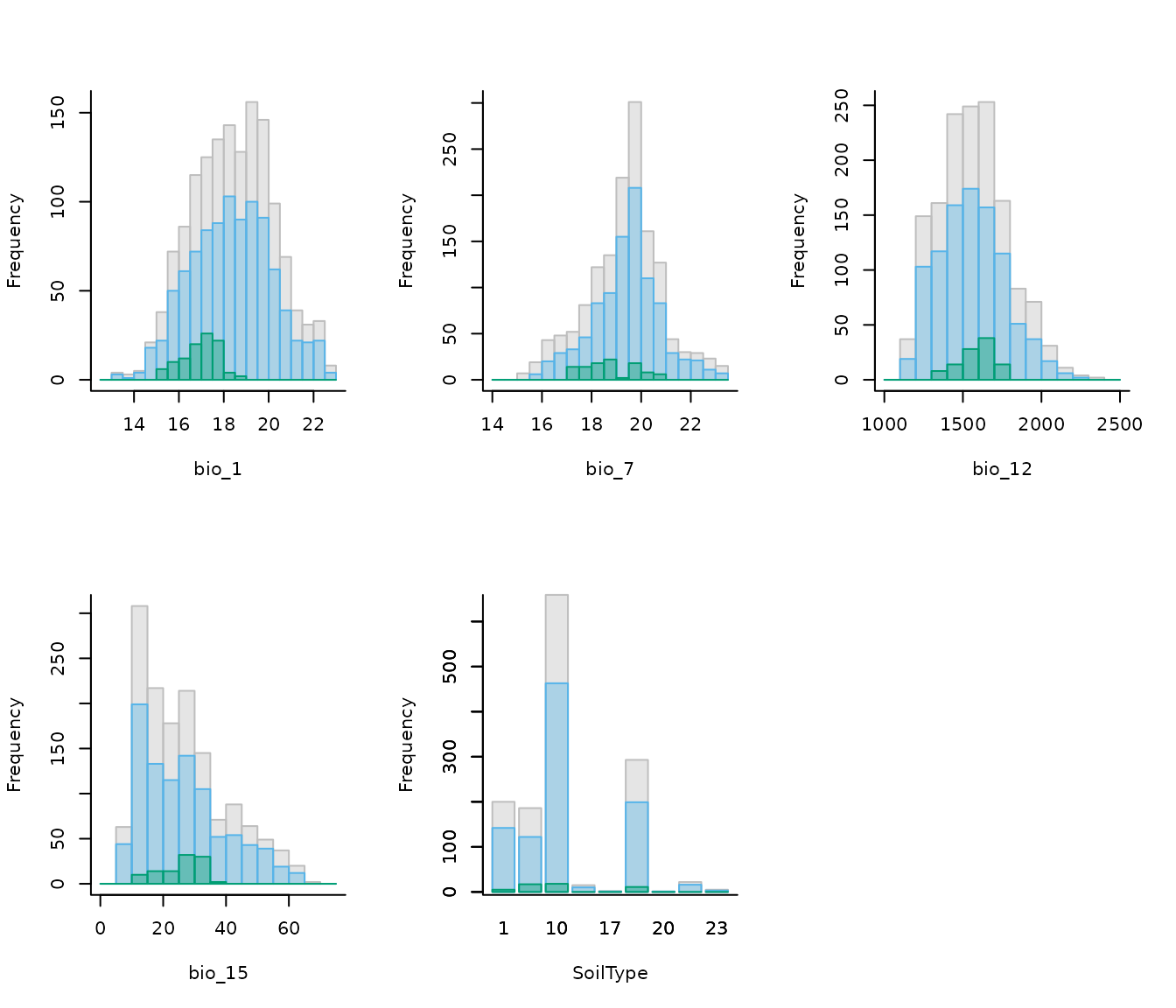
The gray bars represent values across the entire calibration area. Blue bars show values for the background, while green bars display values at presence points (magnified by a factor of 2 for improved visualization). You can customize both the colors and the magnification factor.
Additionally, users can explore the geographic distribution of
occurrence and background points. See full documentation with
help(explore_calibration_geo).
pbg <- explore_calibration_geo(data = d, raster_variables = var[[1]],
plot = TRUE)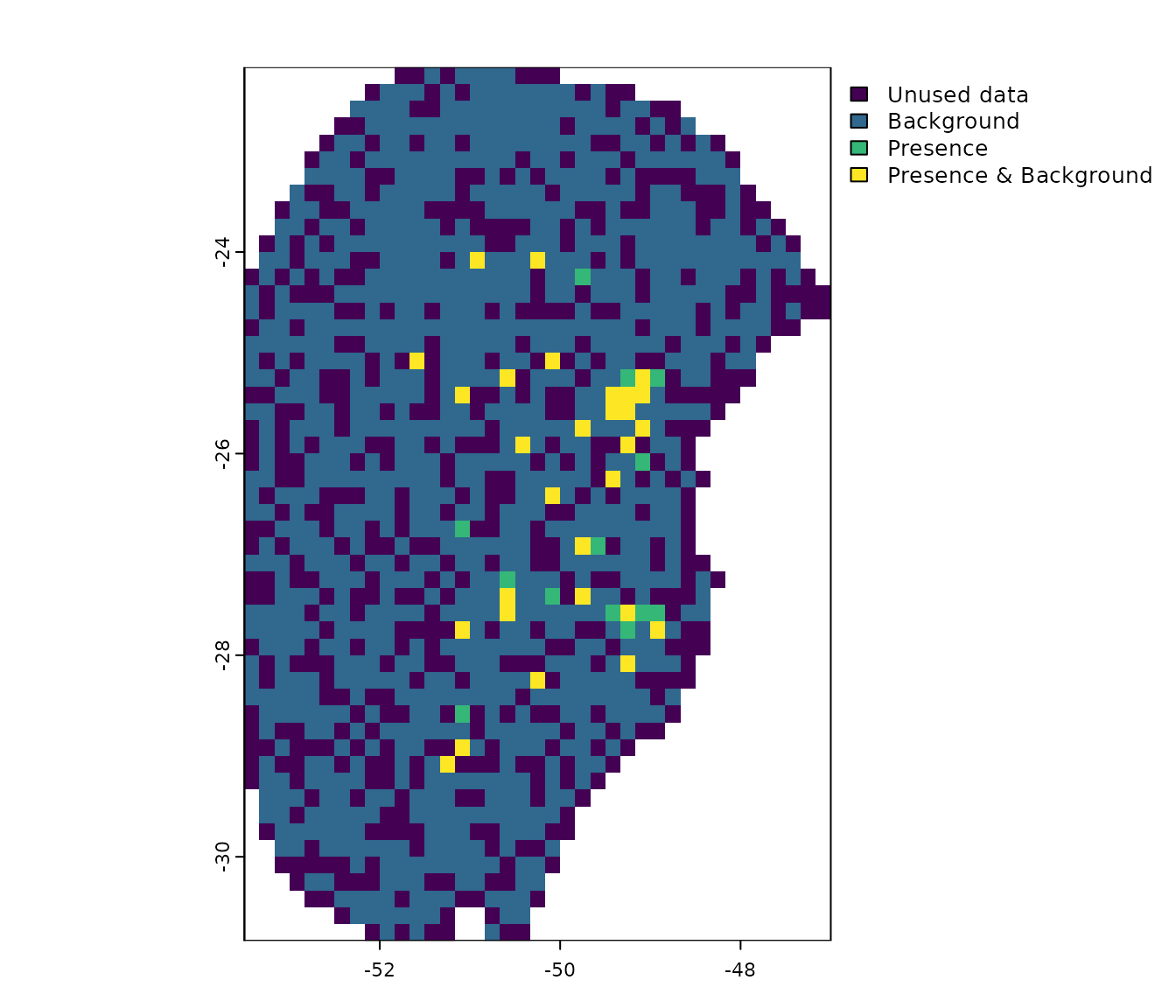
Note that, by default, background points are selected randomly within the calibration area. However, users can influence the spatial distribution of background, increasing or decreasing the probability of selection in certain regions, by providing a bias file (as demonstrated in the next section).
Using a bias file
A bias file is a SpatRaster object that contains values
that will influence the selection of background points within the
calibration area. This can be particularly useful for mitigating
sampling bias, for instance, by incorporating the density of records
from a target group (as discussed in Ponder et
al. 2001, Anderson et
al. 2003, and Barber et
al. 2020).
The bias file must have the same extent, resolution, and number of cells as your raster variables, unless a mask is supplied. If a mask is used, the extent of the bias file should encompass or be larger than the mask extent.
Let’s illustrate this with an example bias file included in the
package. This SpatRaster has lower values in the center and
higher values towards the borders:
# Import a bias file
bias <- rast(system.file("extdata", "bias_file.tif", package = "kuenm2"))
plot(bias)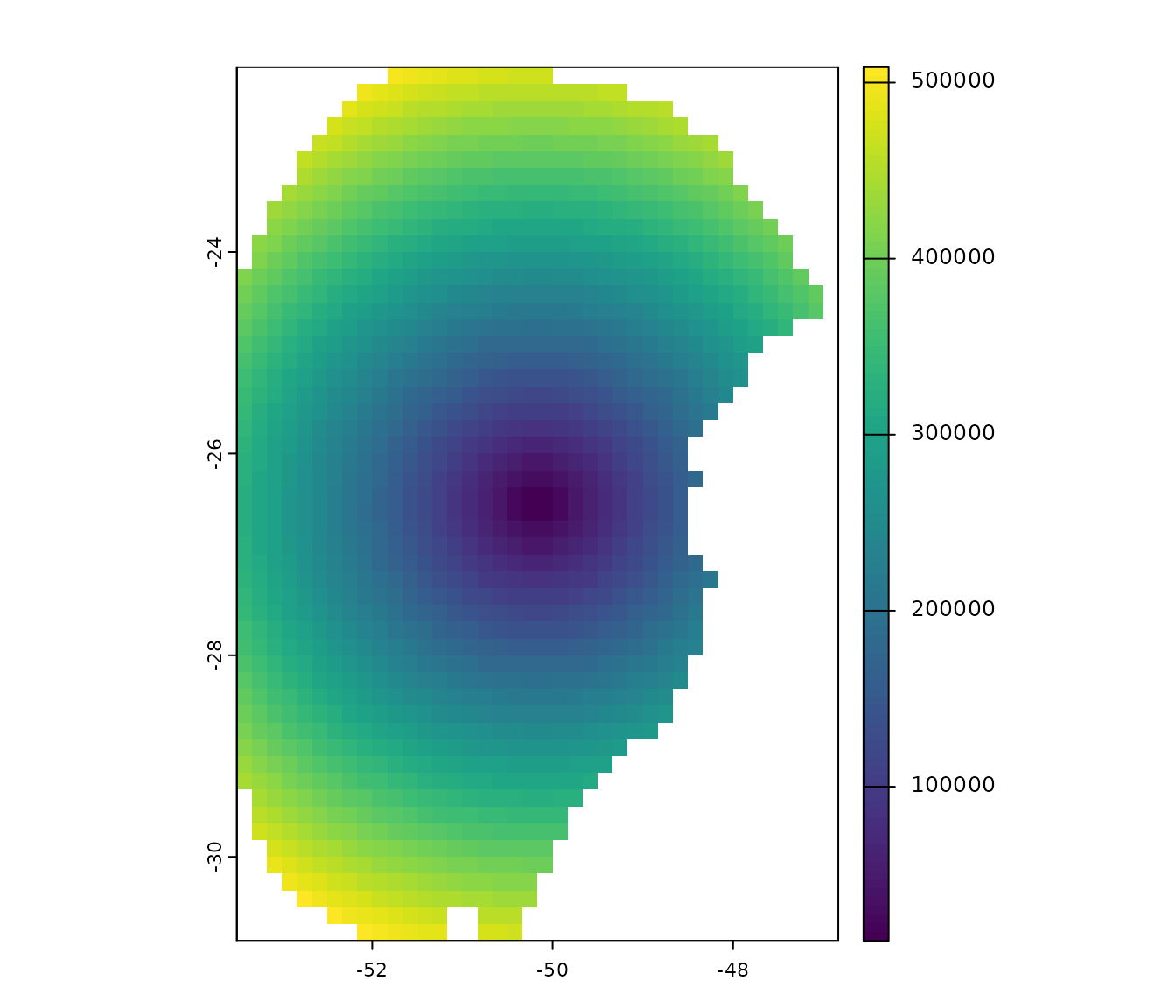
We will now use this bias file to prepare two new datasets: one where the bias effect is “direct” (higher probability in regions with higher bias values) and another where the effect is “inverse” (higher probability in regions with lower bias values):
# Using bias as a direct effect in sampling
d_bias_direct <- prepare_data(algorithm = "maxnet",
occ = occ_data,
x = "x", y = "y",
raster_variables = var,
species = "Myrcia hatschbachii",
categorical_variables = "SoilType",
n_background = 1000,
partition_method = "kfolds",
bias_file = bias, bias_effect = "direct", # bias parameters
features = c("l", "q", "p", "lq", "lqp"),
r_multiplier = c(0.1, 1, 2, 3, 5))
#> Warning in handle_missing_data(occ_bg, weights): 57 rows were excluded from
#> database because NAs were found.
# Using bias as an indirect effect in sampling
d_bias_inverse <- prepare_data(algorithm = "maxnet",
occ = occ_data,
x = "x", y = "y",
raster_variables = var,
species = "Myrcia hatschbachii",
categorical_variables = "SoilType",
n_background = 1000,
partition_method = "kfolds",
bias_file = bias, bias_effect = "inverse", # bias parameters
features = c("l", "q", "p", "lq", "lqp"),
r_multiplier = c(0.1, 1, 2, 3, 5))
#> Warning in handle_missing_data(occ_bg, weights): 45 rows were excluded from
#> database because NAs were found.
# Compare background points generated randomly versus with bias effects
## Saving original plotting parameters
original_par <- par(no.readonly = TRUE)
## Adjusting plotting grid
par(mfrow = c(2,2))
## The plots to show sampling bias effects
plot(bias, main = "Bias file")
explore_calibration_geo(d, raster_variables = var[[1]],
main = "Random Background",
plg=list(cex=0.75)) #Decrease size of legend text)
explore_calibration_geo(d_bias_direct, raster_variables = var[[1]],
main = "Direct Bias Effect",
plg=list(cex=0.75)) #Decrease size of legend text)
explore_calibration_geo(d_bias_inverse, raster_variables = var[[1]],
main = "Inverse Bias Effect",
plg=list(cex=0.75)) #Decrease size of legend text)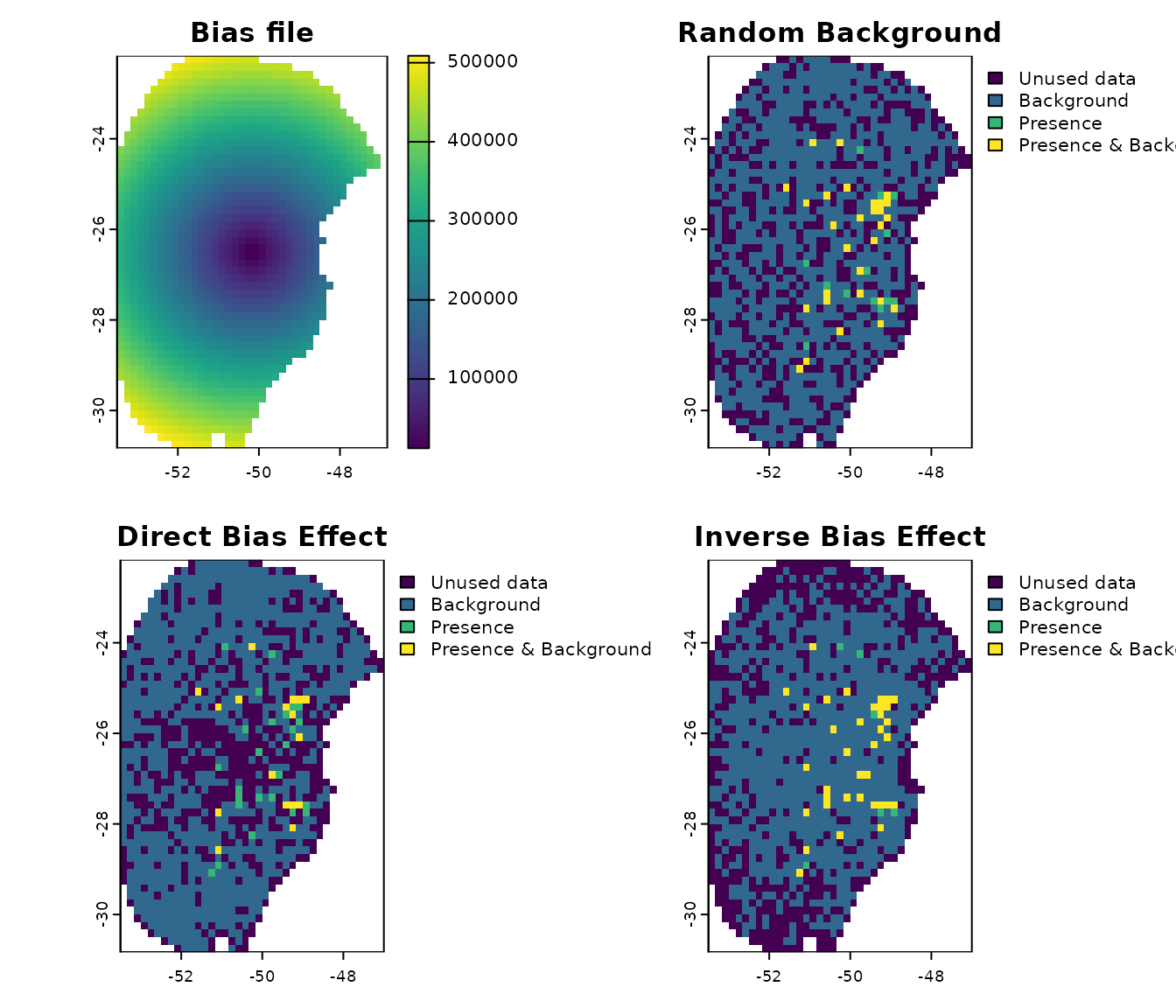
par(original_par) # Reset gridNote that when the bias effect is “direct”, the majority of the background points are sampled from the borders of the calibration area, corresponding to higher bias values. Conversely, with an “inverse” bias effect, most background points are selected from the center, where bias values are lower.
PCA for variables
A common approach in ENM involves summarizing the information from a set of predictor variables into a smaller set of uncorrelated variables using Principal Component Analysis (PCA) (see Cruz-Cardenaz et al. 2014 for an example). In kuenm2 users can perform a PCA internally or use variables that have been externally prepared as PCs.
Internal PCA
kuenm2 can perform all PCA transformations
internally, eliminating the need to prepare the new PC variables for
each scenario of projection. This is particularly advantageous when
projecting model results across multiple time scenarios (e.g., various
Global Climate Models for different future periods). By performing PCA
internally, you only need to store the raw environmental variables
(e.g., bio_1, bio_2, etc.) on your directory,
and the functions will handle the PCA transformation as needed.
Let’s explore how to implement this:
# Prepare data for maxnet models using PCA parameters
d_pca <- prepare_data(algorithm = "maxnet",
occ = occ_data,
x = "x", y = "y",
raster_variables = var,
do_pca = TRUE, center = TRUE, scale = TRUE, # PCA parameters
species = "Myrcia hatschbachii",
categorical_variables = "SoilType",
n_background = 1000,
partition_method = "kfolds",
features = c("l", "q", "p", "lq", "lqp"),
r_multiplier = c(0.1, 1, 2, 3, 5))
#> Warning in handle_missing_data(occ_bg, weights): 43 rows were excluded from
#> database because NAs were found.
print(d_pca)
#> prepared_data object summary
#> ============================
#> Species: Myrcia hatschbachii
#> Number of Records: 1008
#> - Presence: 51
#> - Background: 957
#> Partition Method: kfolds
#> - Number of kfolds: 4
#> Continuous Variables:
#> - bio_1, bio_7, bio_12, bio_15
#> Categorical Variables:
#> - SoilType
#> PCA Information:
#> - Variables included: bio_1, bio_7, bio_12, bio_15
#> - Number of PCA components: 4
#> Weights: No weights provided
#> Calibration Parameters:
#> - Algorithm: maxnet
#> - Number of candidate models: 610
#> - Features classes (responses): l, q, p, lq, lqp
#> - Regularization multipliers: 0.1, 1, 2, 3, 5The elements calibration data and formula grid have now been
generated considering the principal components (PCs). By default, all
continuous variables were included in the PCA, while categorical
variables (e.g., “SoilType”) were excluded. The default settings for the
number of PCs selected retain the axes that collectively explain 95% of
the total variance, and then further filter these, keeping only those
axes that individually explain at least 5% of the variance. These
parameters can be changed using other arguments in the function
prepare_data
# Check calibration data
head(d_pca$calibration_data)
#> pr_bg PC1 PC2 PC3 PC4 SoilType
#> 1 1 1.48690341 1.01252697 0.1180156 -0.09119257 19
#> 2 1 1.46028074 0.17701144 1.1573461 -0.12326796 19
#> 3 1 0.82676494 1.21965795 0.8145129 -0.67588891 6
#> 4 1 0.62680441 0.03967459 0.1525997 0.18784282 1
#> 5 1 0.94584897 0.93302089 1.4382424 -0.03192094 19
#> 6 1 -0.07597437 1.55268331 -0.2007953 -0.98153204 6
# Check formula grid
head(d_pca$formula_grid)
#> ID Formulas R_multiplier Features
#> 1 1 ~PC1 + PC2 -1 0.1 l
#> 2 2 ~PC1 + PC2 -1 1.0 l
#> 3 3 ~PC1 + PC2 -1 2.0 l
#> 4 4 ~PC1 + PC2 -1 3.0 l
#> 5 5 ~PC1 + PC2 -1 5.0 l
#> 6 6 ~PC1 + PC3 -1 5.0 l
# Explore variables distribution
calib_hist_pca <- explore_calibration_hist(data = d_pca, raster_variables = var,
include_m = TRUE, breaks = 7)
plot_explore_calibration(explore_calibration = calib_hist_pca)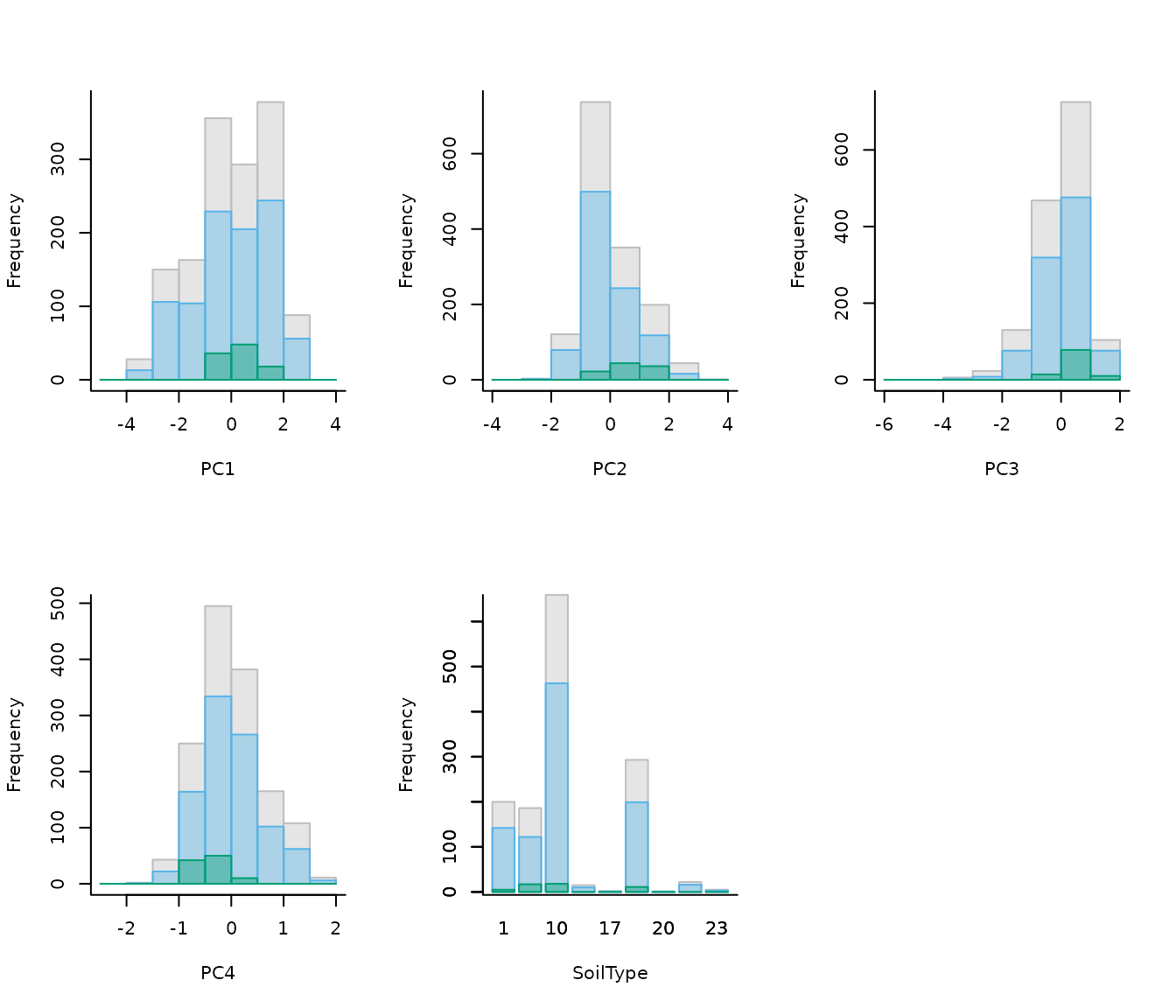
As the PCA was performed internally, the prepared_data
object contains all the necessary information to transform the raw
environmental variables into the required PCs This means that
when predicting or projecting models, users should provide raw raster
variables, and the PCs will be obtained internally in the
function.
External PCA
Alternatively, users can perform a PCA with their data by using the
perform_pca() function, or one of their preference. See
full documentation with help(perform_pca). Se an example
with perform_pca() below:
pca_var <- perform_pca(raster_variables = var, exclude_from_pca = "SoilType",
center = TRUE, scale = TRUE)
# Plot
plot(pca_var$env)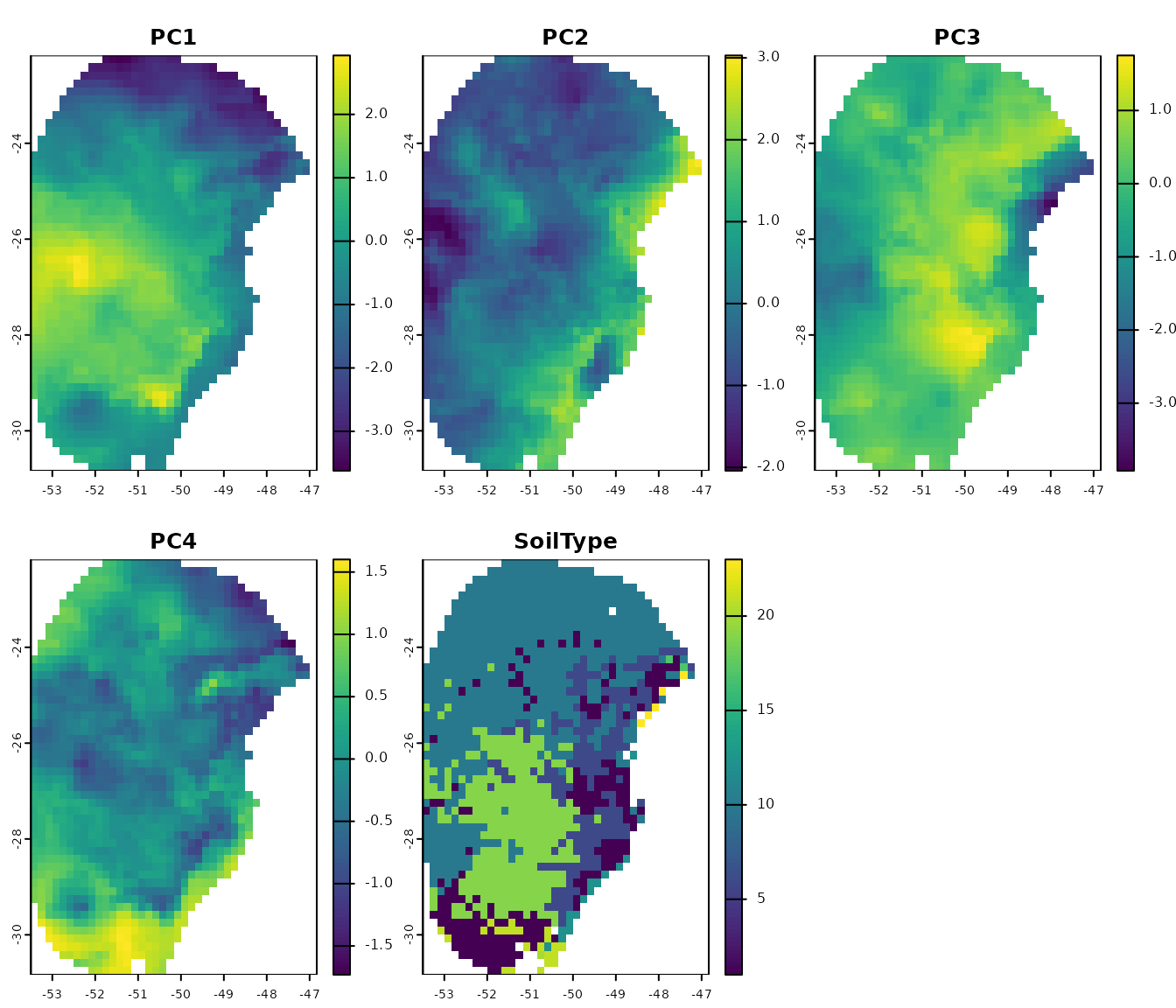
Now, let’s use the PCs generated by perform_pca() to
prepare the data:
# Prepare data for maxnet model using PCA variables
d_pca_extern <- prepare_data(algorithm = "maxnet",
occ = occ_data,
x = "x", y = "y",
raster_variables = pca_var$env, # Output of perform_pca()
do_pca = FALSE, # Set to FALSE because variables are PCs
species = "Myrcia hatschbachii",
categorical_variables = "SoilType",
n_background = 1000,
partition_method = "kfolds",
features = c("l", "q", "p", "lq", "lqp"),
r_multiplier = c(0.1, 1, 2, 3, 5))
#> Warning in handle_missing_data(occ_bg, weights): 43 rows were excluded from
#> database because NAs were found.
print(d_pca_extern)
#> prepared_data object summary
#> ============================
#> Species: Myrcia hatschbachii
#> Number of Records: 1008
#> - Presence: 51
#> - Background: 957
#> Partition Method: kfolds
#> - Number of kfolds: 4
#> Continuous Variables:
#> - PC1, PC2, PC3, PC4
#> Categorical Variables:
#> - SoilType
#> PCA Information: PCA not performed
#> Weights: No weights provided
#> Calibration Parameters:
#> - Algorithm: maxnet
#> - Number of candidate models: 610
#> - Features classes (responses): l, q, p, lq, lqp
#> - Regularization multipliers: 0.1, 1, 2, 3, 5Note that since PCA was performed externally,
do_pca = FALSE is set within the prepare_data
function. This is crucial because setting it to TRUE would
incorrectly apply PCA to variables that are already PCs.
Consequently, the prepared_data object in this scenario
does not store any PCA-related information. This means that when users
predict or project models, they must must provide the
PCs instead of the raw raster variables.
# Check calibration data
head(d_pca_extern$calibration_data)
#> pr_bg PC1 PC2 PC3 PC4 SoilType
#> 1 1 1.48690341 1.01252697 0.1180156 -0.09119257 19
#> 2 1 1.46028074 0.17701144 1.1573461 -0.12326796 19
#> 3 1 0.82676494 1.21965795 0.8145129 -0.67588891 6
#> 4 1 0.62680441 0.03967459 0.1525997 0.18784282 1
#> 5 1 0.94584897 0.93302089 1.4382424 -0.03192094 19
#> 6 1 -0.07597437 1.55268331 -0.2007953 -0.98153204 6
# Check formula grid
head(d_pca_extern$formula_grid)
#> ID Formulas R_multiplier Features
#> 1 1 ~PC1 + PC2 -1 0.1 l
#> 2 2 ~PC1 + PC2 -1 1.0 l
#> 3 3 ~PC1 + PC2 -1 2.0 l
#> 4 4 ~PC1 + PC2 -1 3.0 l
#> 5 5 ~PC1 + PC2 -1 5.0 l
#> 6 6 ~PC1 + PC3 -1 5.0 lPrepare user pre-processed data
If users already have data that has been prepared for calibration,
they can use the prepare_user_data() function to create the
object required for model calibration. User-prepared calibration data
must be a data.frame that includes a column indicating
presence (1) and background (0)
records, along with columns with values for each of your
variables. The package includes an example of such a
data.frame for reference. See an example of its use
below:
data("user_data", package = "kuenm2")
head(user_data)
#> pr_bg bio_1 bio_7 bio_12 bio_15 SoilType
#> 1 1 16.49046 18.66075 1778 12.96107 19
#> 2 1 15.46644 19.65775 1560 14.14697 19
#> 3 1 15.70560 17.99450 1652 23.27548 6
#> 4 1 17.78899 19.55600 1597 18.91694 1
#> 5 1 15.50116 18.30750 1497 15.39440 19
#> 7 1 17.42421 17.25875 1760 34.17664 6The prepare_user_data() function operates similarly to
prepare_data(), but with a key difference: instead of
requiring a data.frame of occurrence coordinates and a
SpatRaster of predictor variables, it takes your already
prepared user data.frame (see below). See full
documentation with help(prepare_user_data).
# Prepare data for maxnet model
data_user <- prepare_user_data(algorithm = "maxnet",
user_data = user_data, # user-prepared data.frame
pr_bg = "pr_bg",
species = "Myrcia hatschbachii",
categorical_variables = "SoilType",
partition_method = "bootstrap",
features = c("l", "q", "p", "lq", "lqp"),
r_multiplier = c(0.1, 1, 2, 3, 5))
data_user
#> prepared_data object summary
#> ============================
#> Species: Myrcia hatschbachii
#> Number of Records: 527
#> - Presence: 51
#> - Background: 476
#> Partition Method: bootstrap
#> - Number of replicates:
#> - Train proportion: 0.7
#> Continuous Variables:
#> - bio_1, bio_7, bio_12, bio_15
#> Categorical Variables:
#> - SoilType
#> PCA Information: PCA not performed
#> Weights: No weights provided
#> Calibration Parameters:
#> - Algorithm: maxnet
#> - Number of candidate models: 610
#> - Features classes (responses): l, q, p, lq, lqp
#> - Regularization multipliers: 0.1, 1, 2, 3, 5This function also allows you to provide a list of index identifying
test points for cross-validation to be used during model calibration. If
user_folds is NULL, the function will automatically
partition the data according to the specified
partition_method, n_partitions, and
train_proportion.
Using Custom Data Partitions
The functions prepare_data() and
prepare_user_data() in the kuenm2 package
include four built-in methods for randomly partitioning data:
“kfolds”: Splits the dataset into K subsets (folds) of approximately equal size. In each partition, one fold is used as the test set, while the remaining folds are combined to form the training set.
“bootstrap”: Creates the training dataset by sampling observations from the original dataset with replacement (i.e., the same observation can be selected multiple times). The test set consists of the observations that were not selected in that specific replicate.
“subsample”: Similar to bootstrap, but the training set is created by sampling without replacement (i.e., each observation is selected at most once). The test set includes the observations not selected for training.
Other methods for data partitioning are also available, including
those based on spatial rules (Radosavljevic
and Anderson, 2014). Although kuenm2 does not currently
implement spatial partitioning techniques, it is possible to apply them
using other R packages and then incorporate the resulting partitions
into the prepared_data object for model calibration within
the kuenm2 framework.
The ENMeval
and flexsdm
R packages offers some options. Below, we provide examples on how to
split the data using this packages and include this partition into the
prepared_data object to run with kuenm2.
Spatial Partitioning with ENMeval
The ENMeval package (Kass et al. 2021) provides three spatial partitioning methods:
Spatial block: Divides occurrence data into four groups based on latitude and longitude lines, aiming for groups of roughly equal size.
Checkerboard 1 (basic): Generates a checkerboard grid over the study area and assigns points to groups based on their location in the grid.
Checkerboard 2 (hierarchical): Aggregates the input raster at two hierarchical spatial scales using specified aggregation factors. Points are then grouped based on their position in the resulting hierarchical checkerboard.
As an example, let’s use the spatial block method to
partition our dataset. For this example, we will use the object
d, which corresponds to the prepared_data
created in previous steps.
# Install ENMeval if not already installed
if(!require("ENMeval")){
install.packages("ENMeval")
}
# Extract calibration data from the prepared_data object and separate presence and background records
calib_occ <- d$data_xy[d$calibration_data$pr_bg == 1,] #Presences
calib_bg <- d$data_xy[d$calibration_data$pr_bg == 0,] #Background
# Apply spatial block partitioning using ENMeval
enmeval_block <- get.block(occs = calib_occ, bg = calib_bg)
# Inspect the structure of the output
str(enmeval_block)
#> List of 2
#> $ occs.grp: num [1:51] 1 1 2 2 1 4 2 2 4 2 ...
#> $ bg.grp : num [1:957] 2 4 4 1 3 3 3 1 1 3 ...Note that the output of get.block() is a list with two
elements: occs.grp and bg.grp. The
occs.grp vector has the same length as the number of
occurrence records, and bg.grp has the same length as the
background points. Both vectors contain numeric values from 1 to 4,
indicating the spatial group to which each point belongs. For example,
an occurrence labeled with 1 belongs to the first spatial
block, 2 to the second block, and so on.
In contrast, kuenm2 stores partitioned data in a
different format: it expects a list of vectors, where
each element corresponds to a partition and contains the indices
of the test points for that partition. The indices include both
presence and background points. During model evaluation for each
partition, only the presence test points are used. The background
points, although present in the dataset, are simply excluded from the
calibration process.
str(d$part_data)
#> List of 4
#> $ Partition_1: num [1:253] 1 3 12 13 14 20 24 25 34 43 ...
#> $ Partition_2: num [1:252] 5 7 8 10 15 17 23 27 31 33 ...
#> $ Partition_3: num [1:251] 4 9 19 21 28 29 30 32 35 36 ...
#> $ Partition_4: num [1:252] 2 6 11 16 18 22 26 37 38 40 ...We can convert the spatial group information stored in
enmeval_block into a format compatible with
kuenm2:
# Identify unique spatial blocks
id_blocks <- sort(unique(unlist(enmeval_block)))
# Create a list of test indices for each spatial block
new_part_data <- lapply(id_blocks, function(i) {
# Indices of occurrence records in group i
rep_i_presence <- which(enmeval_block$occs.grp == i)
# Indices of background records in group i
rep_i_bg <- which(enmeval_block$bg.grp == i)
# To get the right indices, we need to sum the number of records
rep_i_bg <- rep_i_bg + nrow(occ_data)
# Combine presence and background indices for the test set
c(rep_i_presence, rep_i_bg)
})
# Assign names to each partition
names(new_part_data) <- paste0("Partition_", id_blocks)
# Inspect the structure of the new partitioned data
str(new_part_data)
#> List of 4
#> $ Partition_1: int [1:406] 1 2 5 12 13 14 15 29 31 42 ...
#> $ Partition_2: int [1:108] 3 4 7 8 10 16 18 22 27 36 ...
#> $ Partition_3: int [1:367] 11 19 20 21 24 26 30 33 34 38 ...
#> $ Partition_4: int [1:127] 6 9 17 23 25 28 32 35 37 39 ...We now have a list containing four vectors, each storing the indices
of test data defined using the get.block() function from
the ENMeval package. The final step is to replace the
original part_data in the prepared_data object
with new_part_data. We should also update the partitioning
method to reflect this change.
# Replace the original partition data with the new spatial blocks
d_spatial_block <- d
d_spatial_block$part_data <- new_part_data
# Update the partitioning method to reflect the new approach
d_spatial_block$partition_method <- "Spatial block (ENMeval)" # You can use any descriptive name
# Print the updated prepared_data object
print(d_spatial_block)
#> prepared_data object summary
#> ============================
#> Species: Myrcia hatschbachii
#> Number of Records: 1008
#> - Presence: 51
#> - Background: 957
#> Partition Method: Spatial block (ENMeval)
#> Continuous Variables:
#> - bio_1, bio_7, bio_12, bio_15
#> Categorical Variables:
#> - SoilType
#> PCA Information: PCA not performed
#> Weights: No weights provided
#> Calibration Parameters:
#> - Algorithm: maxnet
#> - Number of candidate models: 300
#> - Features classes (responses): l, q, lq, lqp
#> - Regularization multipliers: 0.1, 2, 1Spatial Partitioning with flexsdm
The flexsdm (Velazco
et al. 2022) offers similar spatial partitioning methods. However,
it determines the number or size of bands or blocks based on spatial
autocorrelation, environmental similarity, and the number of presence
and background records within each partition. For more details, please
visit the package
website.
As an example, we will use the Spatial Block method, which evaluates different raster cell sizes to identify the optimal block configuration for the dataset.
# Install flexsdm if not already installed
if (!require("flexsdm")) {
install.packages("flexsdm")
}
# Combine calibration data with spatial coordinates
calib_data <- cbind(d$data_xy, d$calibration_data)
# Split data in test and train using flexsdm
flexsdm_block <- part_sblock(env_layer = var, data = calib_data,
x = "x", y = "y",
pr_ab = "pr_bg", min_res_mult = 1,
max_res_mult = 500, num_grids = 30, n_part = 4,
prop = 0.5)
#> The following grid cell sizes will be tested:
#> 0.17 | 3.03 | 5.9 | 8.77 | 11.64 | 14.51 | 17.37 | 20.24 | 23.11 | 25.98 |
#> 28.84 | 31.71 | 34.58 | 37.45 | 40.32 | 43.18 | 46.05 | 48.92 | 51.79 |
#> 54.66 | 57.52 | 60.39 | 63.26 | 66.13 | 68.99 | 71.86 | 74.73 | 77.6 |
#> 80.47 | 83.33
#>
#> Creating basic raster mask...
#>
#> Searching for the optimal grid size...
# See the structure of the object
str(flexsdm_block$part)
#> Classes ‘tbl_df’, ‘tbl’ and 'data.frame': 1008 obs. of 4 variables:
#> $ x : num -51.3 -50.6 -49.3 -49.8 -50.2 ...
#> $ y : num -29 -27.6 -27.8 -26.9 -28.2 ...
#> $ pr_ab: num 1 1 1 1 1 1 1 1 1 1 ...
#> $ .part: int 3 1 3 2 3 3 1 4 4 3 ...The output from flexsdm differs from that of
ENMeval. Instead of returning a list with vectors of
spatial group IDs for occurrences and background points separately,
flexsdm appends the group assignments as a new column
within the part element of its output.
As with the ENMeval example, we can transform the spatial group
information stored in flexsdm_block into a format
compatible with kuenm2:
# Identify unique spatial blocks from flexsdm output
id_blocks <- sort(unique(flexsdm_block$part$.part))
# Create a list of test indices for each spatial block
new_part_data_flexsdm <- lapply(id_blocks, function(i) {
# Indices of occurrences and background points in group i
which(flexsdm_block$part$.part == i)
})
# Assign names to each partition partition
names(new_part_data_flexsdm) <- paste0("Partition_", id_blocks)
# Inspect the structure of the new partitioned data
str(new_part_data_flexsdm)
#> List of 4
#> $ Partition_1: int [1:248] 2 7 13 21 24 25 26 27 36 40 ...
#> $ Partition_2: int [1:266] 4 12 15 17 18 19 29 31 32 33 ...
#> $ Partition_3: int [1:247] 1 3 5 6 10 16 20 28 34 35 ...
#> $ Partition_4: int [1:247] 8 9 11 14 22 23 30 37 38 41 ...
# Replace the partition data in the prepared_data object
d_block_flexsdm <- d
d_block_flexsdm$part_data <- new_part_data_flexsdm
# Update the partition method description
d_block_flexsdm$partition_method <- "Spatial block (flexsdm)" # You can choose any descriptive name
# Display the updated prepared_data object
print(d_block_flexsdm)
#> prepared_data object summary
#> ============================
#> Species: Myrcia hatschbachii
#> Number of Records: 1008
#> - Presence: 51
#> - Background: 957
#> Partition Method: Spatial block (flexsdm)
#> Continuous Variables:
#> - bio_1, bio_7, bio_12, bio_15
#> Categorical Variables:
#> - SoilType
#> PCA Information: PCA not performed
#> Weights: No weights provided
#> Calibration Parameters:
#> - Algorithm: maxnet
#> - Number of candidate models: 300
#> - Features classes (responses): l, q, lq, lqp
#> - Regularization multipliers: 0.1, 2, 1Analysis of extrapolation risks in partitions
When we partition a dataset, some test records may fall in locations
with environmental conditions not represented in the training data. As a
result, model evaluation can occur under extrapolation, meaning the
model is being tested on conditions outside the range it was calibrated
on. The risk of extrapolation increases when spatial blocks are used for
data partitioning. This is because environmental conditions in one
spatial block may differ significantly from those in others. As an
example, let’s use the prepared_data object that was
partitioned using spatial blocks from the ENMeval
package.
The explore_partition_extrapolation() function
calculates environmental dissimilarity and detects non-analogous
conditions between training and test data for each partition using the
MOP (Mobility-Oriented Parity) metric (Owens et
al. 2013). It requires a prepared_data object. To
visualize the spatial distribution of test data falling within or
outside the training data range, you must also provide the
raster_variables used during data preparation.
By default, the function uses all training data (presences and
backgrounds) to define the environmental range but computes MOP only for
test presence records. To also compute MOP for test background points,
set include_test_background = TRUE:
mop_partition <- explore_partition_extrapolation(data = d_spatial_block,
include_test_background = T,
raster_variables = var)The function returned a data.frame containing:
- mop_distance: MOP distances;
- inside_range an indicator of whether environmental conditions at each test record fall within the training range;
- n_var_out: the number of variables outside the training range;
- towards_low and towards_high : the names of variables with values lower or higher than the training range;
-
SoilType: because the
prepared_dataobject includes a categorical variable, it also contains a column indicating which values in the testing data were not present in the training data.
# Highlight some partitions with extrapolation risks
head(mop_partition$Mop_results[mop_partition$Mop_results$n_var_out > 1, ])
#> Partition pr_bg x y mop_distance inside_range n_var_out
#> 87 Partition_1 0 -50.75000 -30.75000 12.57765 FALSE 2
#> 129 Partition_1 0 -51.75000 -29.91667 10.91112 FALSE 2
#> 193 Partition_1 0 -53.41667 -27.08333 43.59743 FALSE 2
#> 199 Partition_1 0 -52.41667 -26.75000 171.15194 FALSE 2
#> 252 Partition_1 0 -51.58333 -30.75000 15.76803 FALSE 2
#> 261 Partition_1 0 -53.25000 -27.08333 43.59052 FALSE 2
#> towards_low towards_high SoilType
#> 87 bio_15 <NA> 21
#> 129 bio_15 <NA> 21
#> 193 bio_15 bio_7 <NA>
#> 199 bio_15 bio_12 <NA>
#> 252 bio_15 <NA> 21
#> 261 bio_15 bio_7 <NA>Because we set return_raster_result = TRUE, the function
also returned a SpatRaster showing the spatial distribution
of training presences and testing data:
plot(mop_partition$Spatial_results,
mar = c(3, 3, 2, 8), #Adjust margin
plg=list(cex=0.75)) #Decrease size of legend text)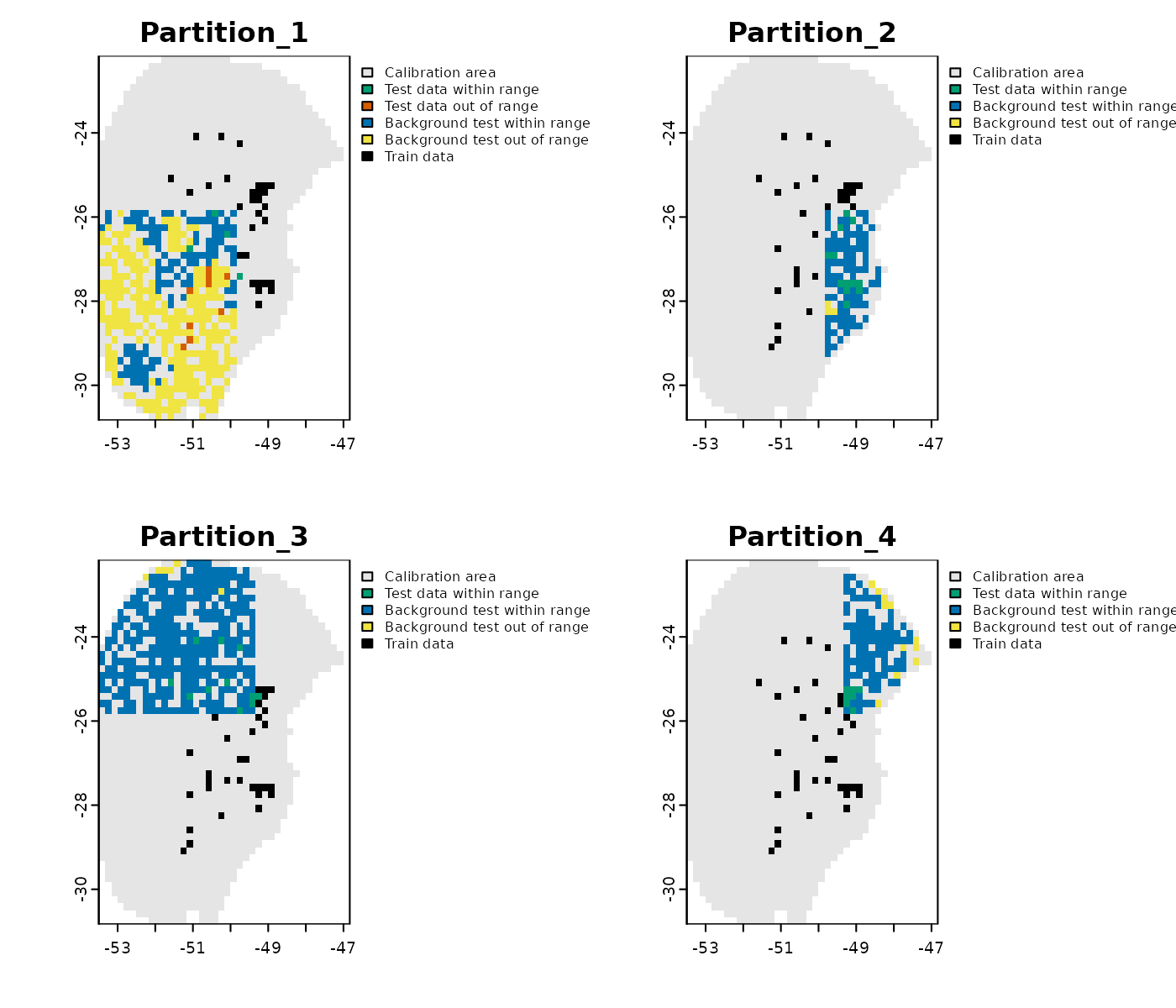
Note that in Partition 1, some test records fall in locations with environmental conditions not represented in the training data. This partition also includes many background points representing conditions that were not used to calibrate its models.
However, it’s important to mention that this function only identifies environmental conditions that fall outside the range of the training data. To fully evaluate the risk of extrapolation, you should look at the MOP results together with the response curves. This combined analysis helps to see how the model behaves under those conditions. For more details, check the Exploring Model Uncertainty and Variability vignette.
Saving a prepared_data object
The prepared_data object is crucial for the next step in
the ENM workflow in kuenm2, model calibration. As this
object is essentially a list, users can save it to a local directory
using saveRDS(). Saving the object facilitates loading it
back into your R session later using readRDS(). See an
example below: